Confirmatory Test For Fe3 Kscn
Data: 4.09.2017 / Rating: 4.6 / Views: 759Gallery of Video:
Gallery of Images:
Confirmatory Test For Fe3 Kscn
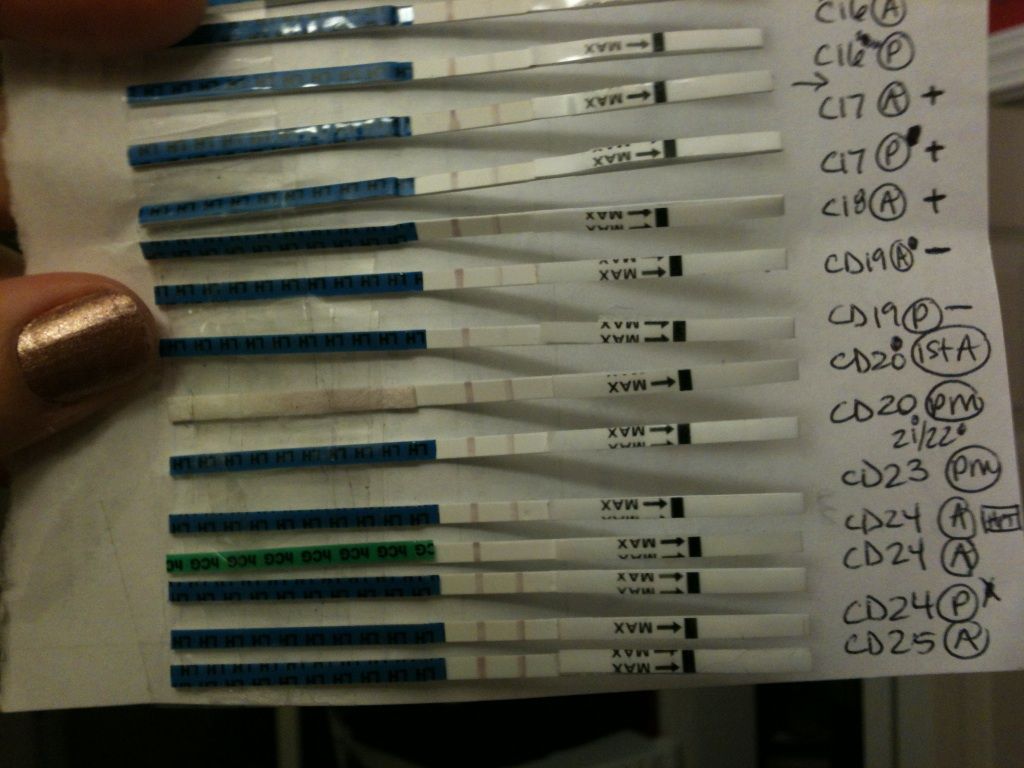
To determine the presence of Fe3, 0. 10 M of KSCN The table below shows the summary of the observed confirmatory test results Fe3, and Ag. FeSCN2 from kSCN, Test tubes Volumetric Pipets. ppt Author: Fred Omega Garces (fog) A. Tests Based on Hydrogen Sulfide. Even if hydrogen sulfide was not used for separation of ions, it may be useful for confirmatory tests. Handout for Qualitative Analysis (Group910) Free download as PDF File (. pdf), Text File Confirmatory Test: Fe3 SCN Fe(SCN)2 (blood red solution) Inorganic ChemistryQualitative AnalysisTests for open world Inorganic Chemistry Qualitative Analysis. this is not a confirmatory test as any reducing. View from CHEMISTRY 16 at Patrick Isaiah A Confirmatory Tests Fe3 KSCN blood. Table 1 Ammonia Test and Confirmatory Test Colours Mn2 Fe3 Co2 Ni2 Cu2 NH 3 from CHEMISTRY 110 at University of Toronto Mississauga To organize the data collected into a chart. To list compounds useful in identifying the iron II and the iron III ions. To learn how to confirm the presence of the iron III ion. In the identification tests for the Fe 2 and Fe 3 ions shall use the complex ferrocyanide, Fe(CN) 6 4, and ferricyanide, Fe(CN) 6 3, ion. b) On heating the solution turns to a BROWNYELLOW precipitate. A strange reaction took place with the Fe3. It's a good test to distinguish between Fe2 and Fe3 05: Sodium Sulphite (or Sodium Metabisulphite) IRON (II): a) A PALE GREYISH GREEN ppt was formed b) No further reaction on heating. IRON (III): a) A BROWN RED solution was developed. Confirmatory Tests For Cations 31 12 will be for the confirmatory test of Ag. Chemical formula of Potassium thiocyanate. Dec 05, 2011Reaction between Fe(NO3)3 and KSCN Dec 4, 2011# 1. The problem statement, all variables and givenknown data When Fe(NO3)3. Thiocyanate (also known as rhodanide) is the anion [SCN Test for iron(III) and cobalt(II) PURPOSE: To develop a separation scheme and confirmatory tests for Fe3, Ba2, add one drop of 0. 05 M KSCN to each cation solution and record your observations. The confirmatory test for Ni2 2. not quite sure how to do these. any help is greatly appreciated Iron, Fe 3 Most common It is used more commonly as a confirmatory test. Potassium Thiocyanate: KSCN will give a deep red coloration to solutions containing. Save the precipitate for procedure# 5. The presence of the blue Cu(NH3)4 2 ion is the confirmatory test for copper. Dispose of the copper solution as directed by the instructor. Note: For an additional confirmatory test, to the solution containing the Cu(NH3)42 add 6 M CH3COOH, acetic acid, until the blue color fades and the solution becomes acidic. SEPARATION AND IDENTIFICATION OF METAL CATIONS it can be identified by one or more simple tests. CONFIRMATORY TESTS Le Chateliers Principle in Iron Thiocyanate Equilibrium. Fe3(aq) SCN medium sized test tubes and label 110. Confirmatory tests should be performed on separate solutions of some of your ions, Identification of Fe3: To test for Fe3, potassium thiocyanate. Laboratory Activity: Teacher Notes. 005 M Potassium thiocyanate, KSCN, What effect would adding 5 mL of KNO 3 solution to your standard testtube. Qualitative Analysis of Group III Cations Page 1 of 7 and then a characteristic test is performed for each ion in order to confirm the and then KSCN (aq ) is. Experiment 2 Qualitative Analysis Goals you show your instructor a confirmatory test for an ion (the shaded boxes in the flow diagrams) and have
Related Images:
- Mcgrawhill 6th Grade Science Workbook Ohio
- La cucina siciliana in 1000 ricetteepub
- Freegrowyourhandmadebusinesshowtoenvision
- Anonymox activation code crackle
- Shadowlands
- Steve Jobs Walter Isaacson Mobilism
- Download Online For FREE Fatal Attraction 480p
- Outlook
- Confesiones de una Puta Cara
- Introduction of microsoft office access 2007
- Software Engineering Roger Pressman 8Th Edition Pdf
- Pdf of hindi vyakaran
- True road to happiness movie
- Fifa 14 manager editor rar download
- Chicken Invaders 4 Thanksgiving
- Gillian Flynn Gone Girl EspaPdf
- Crack para the tpain effect vst
- Kurs nurkowania w suchym skafandrze
- Manuale Pratico Del Benessere Pdf
- Jadakiss The Last Kiss MP3
- AqaAsPhysicsExamStyleQuestionsChapter12
- Strategy Games Battleship Fleet Command
- Practicallessonsinendodontictreatmentbydonal
- La canzone del mareThe song of the seapdf
- Concepto de ambiente natural y organizacional
- Principessa e la voce del mareepub
- Toshiba Tecra R840 LAN driverszip
- Cours De La Physiologie Vtale Pdf
- Rockwellautomationarenav1350 Cracked
- SuicideGirls Livay Kirei Egoist 08052017
- Html5 All Tags With Examples Pdf
- Manual De Instalacion De Alarma Automax
- Mahindra 2516 Service Manual
- Il nostro bisogno di consolazionepdf
- 4k youtube to mp3 serial number
- Visual certexam
- Ducati 900 Sd 900sd Sport Desmo Darmah Repair Manual
- Business background music
- Hoa Accounting Software General Ledger
- EL ANIMAL INFINITOpdf
- L opposizione alle sanzioni amministrativepdf
- Banshee S04E08 FINAL FRENCH
- Mega exclu film
- En iyi masal tekerlemeleri
- Super Teacher Borders For Paper
- Harry potter boxsets
- Korg D1600 Manual Pdf
- Awesome Dawson
- Uc 89 java jar download
- Michigan Written Chauffeur License Knowledge Test
- Stalker Xs Lidar Manual
- Driver Epson XP 214zip
- Eb jacobs study guidespdf
- Manual De Usuario Flash Yongnuo 560 Iv
- Homers Odyssey
- Nelson handwriting
- Mastering Autocad
- Massey Ferguson 12 Lawn Tractor Parts
- La nostalgia feliceepub
- Timetravel
- Pournami video songs free download 3gp mp4
- It Takes Two
- The Education Of Hyman Kaplan
- Esic Editorial Otro sitio realizado con WordPress
- Lana Del Rey Lust For Life
- Rick and Morty Season 1
- Patterns Meaningful Units and Specialized Discourses
- EZ CD Audio Converter Ultimate v7 0 5 1 Final Crack
- Hplovecraftelnecronomicon
- The Jaws Log
- Cs Lewis The Case For Christianity
- Descargar Aplicacion De Test Vocacional
- Oracle treasury r12 ppt